FDA Approves First Gene Therapy For Treatment Of Duchenne Muscular Dystrophy
The U.S. Food and Drug Administration has approved the first gene therapy for the treatment of ambulatory pediatric patients with Duchenne muscular dystrophy (DMD).
The recombinant gene therapy Elevidys is applicable to chldren in the age group of 4 to 5 having this disease with a confirmed mutation in the DMD gene who do not have a pre-existing medical reason preventing treatment with this therapy.
Elevidys manufacturer Sarepta Therapeutics Inc. said continued approval for this indication may be contingent upon verification and description of clinical
benefit in confirmatory trial.
“Today’s approval addresses an urgent unmet medical need and is an important advancement in the treatment of Duchenne muscular dystrophy, a devastating condition with limited treatment options, that leads to a progressive deterioration of an individual’s health over time,” said Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research.
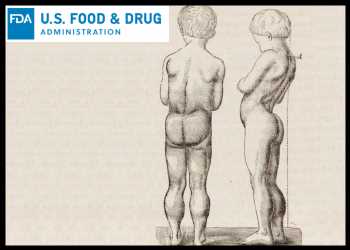
Duchenne muscular dystrophy is a rare and serious genetic condition which worsens over time, leading to weakness and wasting away of the body’s muscles. The disease occurs due to a defective gene that results in absence of dystrophin, a protein that helps keep the body’s muscle cells intact. As a result of this genetic defect, individuals with DMD may have symptoms such as trouble walking and running, falling frequently, fatigue, learning disabilities/difficulties, heart issues as a result of impact on heart muscle functioning, and breathing problems due to weakening of respiratory muscles involved in lung function. Symptoms of muscle weakness associated with DMD typically begin in childhood, often between 3 to 6 years of age.
DMD mainly affects males and in rare cases may affect females. 50,000 people in the U.S. are estimated to have this disease. Most current treatment approaches address the symptoms of the disease, but not its underlying genetic cause.
Elevidys addresses the root genetic cause of Duchenne – mutations in the dystrophin gene that result in the lack of dystrophin protein – by delivering a gene that codes for a shortened form of dystrophin to muscle cells known as ELEVIDYS micro-dystrophin.
Source: Read Full Article