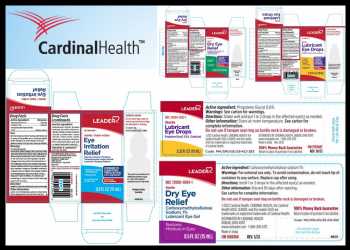
Cardinal Health Recalls Certain LEADER Brand Eye Drops
Cardinal Health, Inc. is recalling certain Leader branded eye drops supplied by Velocity Pharma, LLC citing potential risk of eye infections, according to the US. Food and Drug Administration.
The recall was initiated after the FDA informed the Dublin, Ohio-based company that investigators found insanitary conditions in the manufacturing facility and positive bacterial test results from environmental sampling of critical drug production areas in the facility.
To date, Cardinal Health have received reports of three adverse events related to these recalled products, and the details were shared with its supplier, Velocity Pharma.
The recall involves all lots of over- the-counter or OTC ophthalmic sterile drops supplied by Velocity Pharma to the consumer level.
The products include Eye Irritation Relief; Dry Eye Relief; Lubricant Eye Drops; and Lubricant Eye Drops under LEADER brand that are packaged in various sizes.
The products were distributed across the United States to wholesalers and retailers starting December 12, 2021.
These are used for temporary relief of burning and irritation due to dryness of the eye as well as for use as a protectant against further irritation or to relieve dryness of the eye. Further, the products are also used to relieve redness of the eye due to minor eye irritations. The products are intended to be sterile.
According to the agency, there is a potential risk of eye infections for the patients who use these impacted products that could result in partial vision loss or blindness.
Cardinal Health urged wholesalers, distributors and retailers to cease the distribution of these affected products, and consumers to stop using them. They may return the impacted products to the place of purchase.
In recent incidents, Phoenix, Arizona-based Pharmedica USA LLC in Match recalled Purely Soothing, 15 percent MSM eye drops to the consumer level due to non-sterility.
In mid- September, the FDA had warned eight companies including CVS Health and Walgreens Boots Alliance, Inc. against manufacturing or marketing unapproved ophthalmic drug products in violation of federal law.
For More Such Health News, visit rttnews.com
Source: Read Full Article